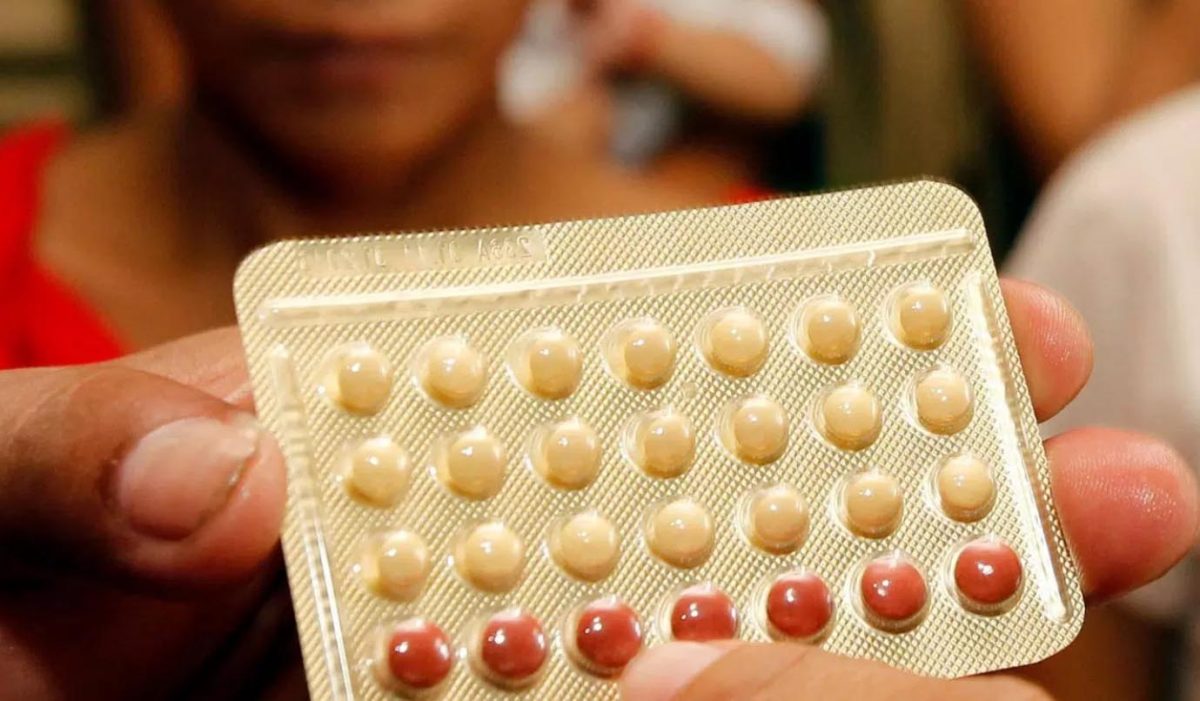
NEW ORLEANS, United States – After taking an experimental male birth control pill for 28 days, 30 men reported no serious side effects and the drug showed signs of decreasing sperm production, according to results of a phase 1 safety test reported Sunday in a poster session at ENDO 2019, the Endocrine Society’s annual meeting in New Orleans.
The purpose of Phase I trials is to gather initial data on a drug’s safety, not to test whether it’s effective. In fact, the duration of the trial was insufficient to prove the pill’s effectiveness as a contraceptive. That would take 60 to 90 days of use, the researchers said. Instead, the hormone changes seen in the volunteers were “consistent with effective contraception,” according to a news release.
The effects of the drug seemed to fade after the men stopped taking it.
Five men (17 percent) reported a mildly decreased sex drive. Two (7 percent) experienced mild erectile dysfunction although, according to a news release, “sexual activity was not decreased.”
Side effects included fatigue, acne or headache and were seen in four to six men each.
The drug, known as 11-beta-MNTDC, mimics testosterone. The study tested a base dose of 200 milligrams once daily in 14 people. Sixteen men got twice that dose. Another 10 received a dummy pill.
The team, led by Dr. Stephanie Page of the University of Washington School of Medicine in Seattle, is planning longer studies of the drug, also known as 11-beta-methyl-19-nortestosterone dodecylcarbonate.
The same team is testing another potential male birth control pill, dimethandrolone undecanoate, or DMAU. – Reuters